Drugs and Pharmaceuticals: India
(→Nodal agency: National Pharmaceutical Pricing Authority) |
(→Prices of Drugs and pharmaceuticals) |
||
| Line 163: | Line 163: | ||
However, there is a section which feels that price control alone cannot improve access. “Competitive pricing, local production and compulsory licensing will drive down prices“, says Indian Pharmaceutical Alliance secretary general D G Shah. | However, there is a section which feels that price control alone cannot improve access. “Competitive pricing, local production and compulsory licensing will drive down prices“, says Indian Pharmaceutical Alliance secretary general D G Shah. | ||
| + | |||
| + | =Fixed dose combination (FDC) medicines= | ||
| + | ==Health ministry bans 344 FDC drugs, HC lifts ban/ 2016== | ||
| + | [http://epaperbeta.timesofindia.com/Article.aspx?eid=31808&articlexml=344-FDC-drugs-limit-therapy-choices-05122016011034 Sushmi Dey, Dec 5, 2016: The Times of India] | ||
| + | |||
| + | |||
| + | ''' `344 FDC drugs limit therapy choices' ''' | ||
| + | |||
| + | |||
| + | Public health groups have expressed concerns over the Delhi high court order lifting the ban on 344 fixed dose combination (FDC) medicines, imposed by the government. | ||
| + | |||
| + | “This is a huge setback to efforts aimed at bringing a semblance of order into the absolute anarchy that exists in India's pharmaceutical market,“ said a statement from Jan Swasthya Abhiyan. | ||
| + | |||
| + | The health ministry had banned 344 FDC drugs terming them as irrational and raising concerns over their misuse. These included some of the popular pharmaceutical brands such as Pfizer's Corex and Abbott's Phensedyl. However, recently the Delhi HC overturned the government's ban providing a major relief to the pharmaceutical companies. | ||
| + | |||
| + | Public health and advocacy groups have asked the government to appeal against the HC order as it has the potential to impact healthcare cost as well as quality of medicines. Health groups said lifting of the ban seems to be predicated on “perceived procedural issues“ which fundamentally abrogate the right to life and healthcare. | ||
| + | |||
| + | Jan Swasthya Abhiyan said use of FDCs increase cost of medication, exposes populations to a larger array of adverse effects and limits the choice of therapy as they may combine drugs with different dosage schedules. Further, some of the cough syrups in the ban order are primarily being used as addictive substances and not as therapeutic agents. | ||
| + | |||
| + | FDCs include two or more active pharmaceutical ingredients combined in a single dosage form. | ||
| + | |||
| + | “The Delhi High Court order does not appear to address the issues of rationality of the FDCs and the resultant adverse effect on public health,“ it said, asking the government to plug legal and regulatory loopholes so that the ban order can be restored. | ||
| + | |||
| + | “It is of utmost importance that the government, as a custodian of public health, act decisively to de fend it and strengthen regulatory mechanisms,“ JSA said. | ||
| + | |||
| + | The HC verdict has come as a major set back to the government which was planning to ban more FDCs to eliminate irrational combination drugs causing anti-microbial resistance. In some cases the toxicity is so high that it can even lead to failure of organs, officials say. There are also concerns many of these FDCs being available over-the-counter without doctors' prescription, which is leading to their misuse. | ||
| + | |||
| + | While many of these products are sold at the chemists level, officials and health experts say often adverse events are not reported because patients do not come back to doctors unless these drugs are used repetitively , leading to severe problems. | ||
| + | |||
| + | Estimates show around FDCs comprise over 40% of the total Rs 1 lakh crore annual Indian pharmaceutical market. However, only around 16 FDCs are part of the National List of Essential Medicines. | ||
Revision as of 22:23, 19 January 2017


Active pharmaceutical ingredients imported from China, 2016
The Times of India
This is a collection of articles archived for the excellence of their content. Readers will be able to edit existing articles and post new articles directly |
This is a collection of articles archived for the excellence of their content. |
Contents |
Clinical trials
Rules eased/ 2016
The Times of India, Aug 04 2016
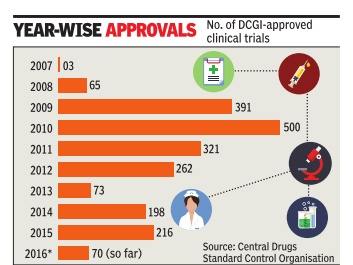
Sushmi Dey Clinical trials may rise as drug regulator eases rules The federal drug regulator has eased norms related to pharmaceutical research, a move that may boost the number of clinical trials but could also raise concerns on patient safety .
Drugs Controller General of India (DCGI) in a notification, said an investigator or researcher can undertake as many trials as approved by the ethics committee instead of the present cap of three. The regulator has also relaxed norms for hospitals or clinical sites undertaking such trials. Through a separate notification, the DCGI has revised the rule that prohibited any hospital with less than 50 beds to take up a trial.
Under the revised norms, the ethics committee has been empowered to decide whether asite is suitable for a trial irrespective of its bed capacity . It suggested the site should have “emergency rescue and care arrangements“.
The ethics committee is a panel of experts which examines the proposal of a pharmaceutical company or a researcher to conduct clinical trials or human experiments for new medicines.
Following random clinical trials and increasing number of deaths during such experiments, the government had earlier imposed a restriction of not more than three clinical trials to be conducted by an investigator.
The number of clinical trials in India went up rapidly around 2008-09. According to data available from the Central Drugs Standard Control Organisation (CDSCO), aro und 65 trials were approved in 2008, whereas it jumped to 391 in 2009 and 500 in 2010. However, following the Supreme Court's intervention, the approvals dropped drastically from 2011onwards.
The changes have cheered the industry . “Ultimately what this translates into is qualitatively better clinical trials as decisions will be guided by which investigator or site is best suited for a particular trial,“ Indian Society of Clinical Research president Suneela Thatte said.
Drugs and pharmaceuticals made in India
Curbs by four EU nations
December 07 2014
Four European countries decided to suspend marketing authorization of 25 drugs, which had undergone tests at the GVK Biosciences facility European Medicines Agency (EMA) did say in a statement that it is reviewing the findings of non-compliance with good clinical practice at the GVK facility and determining its impact on medicines authorized on the basis of studies performed there. Germany , France, Luxembourg and Belgium have already decided to suspend marketing authorizations of these drugs. EMA's Committee for Medicinal Products for Human Use (CHMP) is now identifying, together with member states of the EU, the medicines covered by the inspection findings. French drug regulator ANSM has said on its website that Belgium, Germany , Luxembourg and France decided to suspend the marketing authorizations for the medicinal products concerned. “Although these documents are not essential to the demonstration of bioequivalence, the ANSM decided, as a precaution, to suspend the marketing authorization of 25 marketed generic drugs.
Drugs and pharmaceuticals sold in India
2008- 2014: Drug launches reduced by 80%
Dec 27 2014
New drug launches drop 80% in 6 yrs
Sushmi Dey
Launches of new medicines in India have come down by nearly 80% during 2008-2014 and drug manufacturers blame price regulations and policy uncertainty for making India an unattractive destination for both domestic as well as multinational pharmaceutical companies.
In 2008, 270 new drugs were approved for sale in India, whereas it dropped to 44 and 35 in 2012 and 2013, respectively. In 2014, only 56 new medicines were approved till November, government data shows.
“Most of the big pharmaceutical companies have knocked out India from their list of key markets. The reason is stricter policy measures cutting down on margins, making it less attractive as a business proposition for future growth,” a senior executive in a leading domestic pharmaceutical company said.
A stricter regulatory regime, which not only brings down drug MRPs but also continuously expands span of price control, is cited as the main reason why drug manufacturers are losing their India focus. Industry of ficials also blames looming uncertainty in policy as another reason for companies to delay product launches. Apart from pricing, the pharma industry has been facing hiccups in foreign investment, new drug approvals, as well as clinical trials.
Government and regulators, however, brush aside such concerns. According to a senior official in the National Pharmaceutical Pricing Authority (NPPA), the price regulations are as per the policy and MRPs are fixed based on average price of medicines in that segment. “There is no reason for a particular company to find it unviable when others are making the same drug at a lower price,“ the official said, adding that market surveys show a huge disparity in pric es of similar medicines sold under different brands.
The number of drug approvals peaked in 2008, when as many as 270 new drugs were granted approval, followed by 217 in 2009, 224 in 2010, and 140 in 2011, the data shows.
However, since 2012, when the government released the National Pharmaceutical Pricing Policy bringing in 348 medicine formulations un der price control, the new drug launches started reducing drastically. “The role of NPPA is to implement the policy in letter and spirit and not create confusion leading to instability in the drug industry,“ another senior industry official said.
Recently, many medicines were also found missing from the market following stringent regulatory measures.
Prices of Drugs and pharmaceuticals
Nodal agency: National Pharmaceutical Pricing Authority
The Hindu Business Line, November 26, 2016
S. Srinivasan
NPPA (National Pharmaceutical Pricing Authority), formed in 1997 implements the DPCO (Drug Price Control Order) 2013.
In considering dismantling of the institution, the thought is that price control should be “delinked” from the 370-plus essential drugs. And thereafter price control must be confined to what the government thinks fit. Tell this to the poor people standing in the queue to exchange their demonetised notes. They understand the devastating impact of such a move on their lives.
The argument being given for dismantling price control is that:
a) it inhibits growth of pharma industry and
b) inhibits new investment.
This is a wrong argument and not evidence based. Price control affects only 12 per cent (maximum of Rs. 12,000 crore) of the total domestic market of more than Rs. 1 lakh crore. Out of which less than 50 per cent of the “affected” market, that is only Rs. 6,000 crores, had to actually reduce their prices to the ceiling price fixed by the DPCO. About 88 per cent of the market is out of price control. During the period DPCO 2013 has been in operation, the domestic sales of medicines have increased from Rs. 70,000 crore in 2013 to more than Rs. 100,000 crore as of date. Exports are another Rs. 100,000 crore. So, does the DPCO really inhibit growth?
There is in fact a need to expand the span of price control to cover a range of essential and life-saving drugs as directed by the Supreme Court in its order dated March 10, 2003 in Union of India vs KS Gopinath and Ors. The Supreme Court has clearly held that the government has a Constitutional obligation to ensure the affordability of essential medicines. The AIDAN (All-India Drug Action Network) and Others had impleaded themselves in the matter in 2003 and the case is still before the Supreme Court (Writ Petition (Civil) 423/2003). The current DPCO 2013 is itself an outcome of the above PIL. It is, hence, curious that the Government should discuss the dismantling of the DPCO 2013 when:
a) the matter is sub judice, and
b) when it is against the spirit of Supreme Court directives on the issue.
AIDAN has served a legal notice, citing the case, to those involved with the discussion of dismantling the NPPA (the CEO of the Niti Aayog and the Secretaries of Health, Department of Pharmaceuticals and the Department of Industrial Policy and Promotion).
Pharma industry lobbies are reeling from two recent court orders: one, the refusal by the Bombay High Court to question the legitimacy of the NPPA’s action, under Para 19 of the DPCO 2013, that brought more essential drugs, like antidiabetics and cardiovasculars, under price control. The other order, by the Supreme Court, will result in industry having to pay more than Rs. 4,500 crore for overcharging essential medicines under the previous DPCO. It is no wonder that the very foundation of price control is now being brought to question.
But in the interest of bringing in greater “ease of doing business”, the Government needs to ensure that Parliament and the regulatory requirements are not bypassed, stakeholders are not kept in the dark, and patient interest is not sacrificed.
Trends
Limited rural reach inspite price control measures
The Times of India, Jul 15 2015
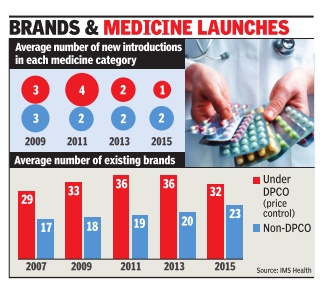
Sushmi Dey
Pharma price control has stunted innovation: Study
Lower cost but rural reach poorer
Consumers may be happy about a cut in medicine bills but the government's price control measures have forced many brands out of the “unviable“ pharmaceutical market, resulting in a drastic slowdown in launches over the last five years. From an average of four new drugs being launched in any specific category in 2011, it was down to a mere one in 2014-15, implying a 75% decline in launches, according to estimates by IMS Health -a leading healthcare market research agency .
Not just that, data collected since 2013, when the new pharmaceutical pricing policy came into place, show a sharp decline in consumption of price-controlled medicines. That's because of a growing push for alternative options outside price control.
With lower margins in price-controlled medicines, there is also less incentive to reach out to rural markets. “For low-income households that are reliant on the government system for healthcare, DPCO (Drugs Price Control Order) will not improve the patient's ability to purchase drugs. This is supported by the fact that no significant penetration of price-controlled molecules in rural markets is visi ble...,“ says a new report, Assessing the Impact of ` Price Control Measures on Access to Medicines in India' by IMS Health.
According to the report, consumption of price-controlled medicines in rural areas dropped by 7% in the past two years, whereas sales of other medicines increased by 5%. It says even in Tier-II and III cities, such medicines have witnessed a muted growth.
The industry argues that the move has failed to achieve the intended objectives of increased affordability and availability because policy measures have impacted tail-end brands more than the leading players.But public health experts brush aside such concerns saying companies manufacture one medicine under several brands with different compositions, and regulations are aimed at bringing them on a par in terms of pricing.
Life-saving medicines: limited competition keeps rates high
Rupali Mukherjee, Critical illness drugs remain unaffordable, Nov 03 2016 : The Times of India
Steps To Cap Prices Of Life-Saving Meds Prove Futile, Limited Competition Keeps Rates High
Market dynamics and limited competition in biosimilars (approved versions of complex biologic drugs) and other life-saving drugs have resulted in little reduction in prices, making them unaffordable for many . Surprisingly , government measures to bring down their prices (through the Drug Price Control Orders, or DPCOs) too have been futile, making them out of reach for patients as treatments often run into lakhs of rupees. Biosimilars are used in cancer therapy , rheumatoid arthritis and other critical illnesses.
Even in the case of medicines for hepatitis B and C like sofosbuvir, tenofovir and entecavir, which were brought under price control, there has been little change in the access scenario. Take the example of two biosimilars -rituximab and trastuzumab -where there is not much change in MRP , even after government brought these drugs under price control in April and May respectively .The National Pharmaceutical Pricing Authority (NPPA) ceiling price for breast cancer drug trastuzumab is Rs 55,812 (440 mg), marginally lower than that charged by companies at around Rs 58,000, while the ceiling price of another cancer drug rituximab (500 mg) is Rs 36,947, close to the MRP of Ristova (innovator Roche's brand) at Rs 37,500.
Ceiling prices are fixed by NPPA based on a simple average of price to retailer (MRP) of products of companies with over 1% market share. “Given this methodology , wherever the innovator has had an almost monopoly position, the price reduction before and after price control has not been significant. Therefore, given the same methodology being in force, a ceiling price revision after two years can be expected to bring about a further reduction consequent to more players participating in the market and offering products at competitive prices,“ K V Subramaniam, president and CEO, Reliance Life Sciences, said.
Leena Menghaney , treatment activist and lawyer, said, “The system devised by NPPA of fixing prices encourages companies to artificially keep prices high, and hence, works against patients and consumer interests. The government has allowed both multinational and generic countries to profiteer out of the disease of people, driving millions of patients to death.“
The cost of production should be taken into consideration, where medicines included in the National List of Essential Medicines (NLEM) are linked to price control. This is because there is evidence that cost of producing certain offpatent registered drugs is cheap, based on cost of active pharmaceutical ingredient (raw material). “The market is not 100% perfect and, at times, anomalies creep in. In certain cases, there are limitations in making drugs affordable,“ an NPPA official said. In the case of hepatitis medicines, pharmacologist Andrew Hill from Liverpool University has calculated the treatment costs for a patient.“The drug entecavir is very cheap, and could be made for only $36 per year, or 10 cents per day . Sofosbuvir can be made for $1 per day , including a profit margin. So the ceiling price fixed by the government seems high,“ Dr Hill told TOI.
“The cost of biosimilars needs to be viewed in the con text of global prices of these expensive biologics which are being provided in India at a fraction of their original cost. The cost of developing a biosimilar ranges between $50-150 million in comparison to a generic which costs around $3-5 million. The scale-up of manufacturing is extremely difficult and expensive,“ Kiran Mazumdar Shaw, CMD, Biocon, said.
However, there is a section which feels that price control alone cannot improve access. “Competitive pricing, local production and compulsory licensing will drive down prices“, says Indian Pharmaceutical Alliance secretary general D G Shah.
Fixed dose combination (FDC) medicines
Health ministry bans 344 FDC drugs, HC lifts ban/ 2016
Sushmi Dey, Dec 5, 2016: The Times of India
`344 FDC drugs limit therapy choices'
Public health groups have expressed concerns over the Delhi high court order lifting the ban on 344 fixed dose combination (FDC) medicines, imposed by the government.
“This is a huge setback to efforts aimed at bringing a semblance of order into the absolute anarchy that exists in India's pharmaceutical market,“ said a statement from Jan Swasthya Abhiyan.
The health ministry had banned 344 FDC drugs terming them as irrational and raising concerns over their misuse. These included some of the popular pharmaceutical brands such as Pfizer's Corex and Abbott's Phensedyl. However, recently the Delhi HC overturned the government's ban providing a major relief to the pharmaceutical companies.
Public health and advocacy groups have asked the government to appeal against the HC order as it has the potential to impact healthcare cost as well as quality of medicines. Health groups said lifting of the ban seems to be predicated on “perceived procedural issues“ which fundamentally abrogate the right to life and healthcare.
Jan Swasthya Abhiyan said use of FDCs increase cost of medication, exposes populations to a larger array of adverse effects and limits the choice of therapy as they may combine drugs with different dosage schedules. Further, some of the cough syrups in the ban order are primarily being used as addictive substances and not as therapeutic agents.
FDCs include two or more active pharmaceutical ingredients combined in a single dosage form.
“The Delhi High Court order does not appear to address the issues of rationality of the FDCs and the resultant adverse effect on public health,“ it said, asking the government to plug legal and regulatory loopholes so that the ban order can be restored.
“It is of utmost importance that the government, as a custodian of public health, act decisively to de fend it and strengthen regulatory mechanisms,“ JSA said.
The HC verdict has come as a major set back to the government which was planning to ban more FDCs to eliminate irrational combination drugs causing anti-microbial resistance. In some cases the toxicity is so high that it can even lead to failure of organs, officials say. There are also concerns many of these FDCs being available over-the-counter without doctors' prescription, which is leading to their misuse.
While many of these products are sold at the chemists level, officials and health experts say often adverse events are not reported because patients do not come back to doctors unless these drugs are used repetitively , leading to severe problems.
Estimates show around FDCs comprise over 40% of the total Rs 1 lakh crore annual Indian pharmaceutical market. However, only around 16 FDCs are part of the National List of Essential Medicines.

